Climántica :: Biblioteca
- Galego
- |
- Castellano
- |
- English
How does solar energy reach us?

The Sun releases energy by means of light passing through the atmosphere. Only one third of the light sent by the Sun to the Earth reaches the planet’s surface, since the atmosphere acts as a very high illuminating radiation filter. Our eyes only perceive a very small part of the light that reaches the Earth, the so-called visible light.
Light travels in the form of waves. In order to understand what a wave is, imagine some particles moving as in the figures below:

Why do we see objects in different colours?
You can see that waves travel by means of oscillations: the first one oscillates more than the second and therefore it is said to have more frequency. Each complete wave oscillation is called a cycle and therefore, the frequency measures the number of cycles completed by the wave per second. Wavelength means a cycle’s length, as you can see in the figures above.
Light does not always produces the same quantity of energy, since the more frequency its wave has the more energy it produces, or the shorter its wave length is.
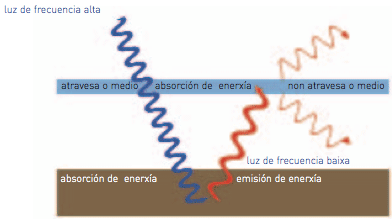
There are two reasons to explain why substances do not let in light: first, because they rebound it off and second, because they absorb it. In this case, the absorbed radiation is transformed into calorific radiation, which it is non-visible (infrared). White objects reflect all the illuminating radiation that reaches them, whereas black objects absorb it and make it into calorific radiation.
You can perceive the colour of things because they have absorbed the complementary colour. That is the reason why you perceive the colour that is not absorbed by them, therefore that reflected (“rebounded off”), allowing us to perceive it.
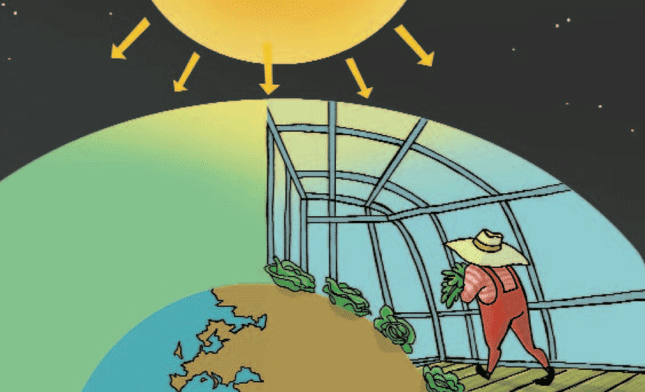
A transparent object such as glass lets in almost all the illuminating radiation but not all the solar radiation because glass is a filter suitable for infrared radiation (calorific radiation). This explanation allows us to understand the introduction and establishment of greenhouses in agriculture.
These agricultural facilities are built when it is necessary to maintain a higher temperature on a farming land’s surface and therefore to avoid the worst consequences of frost on plants. The best greenhouses are made of glass, which is transparent for all colours of light, but they do not let in most infrared or calorific radiation.
If glass were painted red only a part of the visible illuminating radiation could pass through it. In this case the light added to the red light would be absorbed (greenish or bluish colour). From the outside of the greenhouse you could see the non-absorbed light, that is, the reflected (“rebounded off”) red light, which would reach our sight when it is reflected. Part of this non-absorbed red light would pass through the glass and therefore, you could see reddish colours inside the greenhouse. This means that the red glass is not transparent for all the colours of light. In fact, not all the solar radiation passes through the standard glass, but it lets all colours of light in. Therefore, radiation that does not pass through it cannot be perceived by our eyes (infrared or calorific radiation).
While colourless glass lets in almost all the visible radiation, black glass does not; it absorbs all the visible radiation, changing it into calorific radiation (infrared radiation). Therefore, if the greenhouse was made of black glass it would not let in visible radiation and its inside could not be seen. In this case the surface of the black glass would be very hot when the sun shines because it is changing all the illuminating radiation absorbed into infrared radiation. If it was white, it would neither let in most visible light, but in this case not because it absorbs it, but because it reflects it.

Why is it warmer inside than outside a greenhouse?
When the illuminating radiation falls on the black ground of the greenhouse, it is completely absorbed, changing into infrared or calorific radiation, which is released by the Earth towards the atmosphere.
Although it is very easy for the objects that are in the open air to rebound off the infrared radiation (calorific) rebounding it off towards the atmosphere, almost a small part of that irradiated by the ground of a greenhouse can pass through the glass towards the outside atmosphere. Nevertheless, most of it bounces on the inside of the glass, resting inside the greenhouse, which causes the increase of temperature inside.
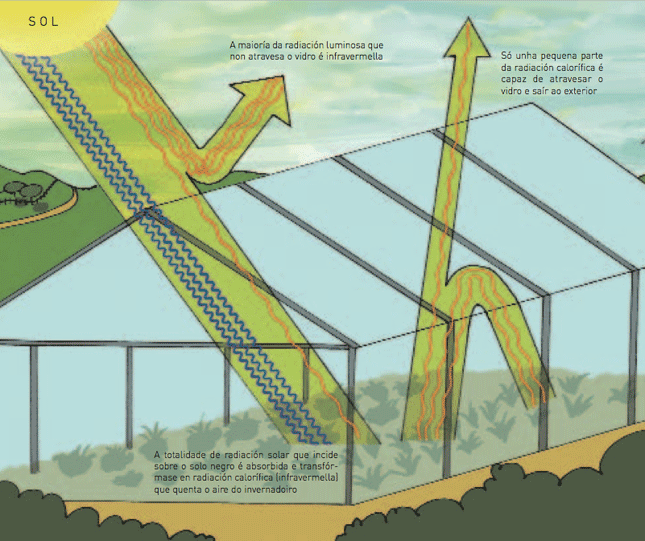
This effect is felt inside the cars or rooms full of windows, mainly in summer sunny days. In some Galician buildings this effect was also used to make winter temperatures warmer, building galleries in sunny spaces.

Greenhouse gases
The Earth’s atmosphere is composed of many gases including the nitrogen, the oxygen and the argon. These gases are quite transparent both for the visible light and the infrared or calorific radiation released by the Earth when it is warm.
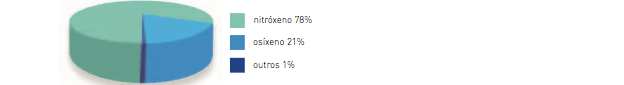
Other gases, representing less than 1 percent, act in a similar way to the glass wall of a greenhouse. The most important are the carbon dioxide (CO2), the methane (CH4) and the nitrous oxide (N2O). Among the greenhouse gases, the carbon dioxide plays an important role as a thermo-regulator, as well as water (H2O) vapour, although in a different way from that of the glass walls of a greenhouse.
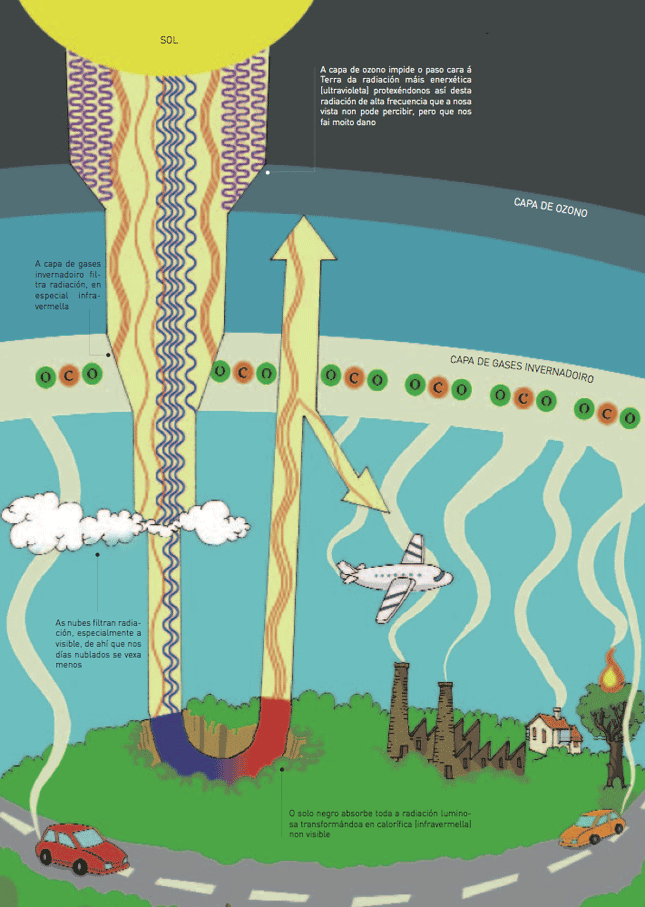
The atmosphere’s filters
When sunlight reaches the atmosphere, some of this energy is filtered through different points by means of absorptions and reflections. The radiation that gives out more energy is not the non-visible one but the ultraviolet one, which is filtered through a very high layer that has a gas called ozone which absorbs it.
Another different filtering layer is that of greenhouse gases, particularly the carbon dioxide. This is different from the former due to its position, the type of gases and the type of radiation that is filtered by them, which is in this case the least energetic: infrared or calorific.
Since this layer does not let a part of the infrared radiation in, the heat released by the Earth, as a result of changing the illuminating radiation absorbed, is rebounded off again towards the Earth’s surface, so that it becomes very similar to the glass walls of a greenhouse, although it acts as if it were a sponge.
Finally, clouds also reflect back and absorb radiation. That is the reason why in cloudy days there is less light. By means of the thermo-regulating action of water, the water vapour layer is also relevant to the thermal regulation that allows life on Earth, although in a different way from the carbon dioxide.
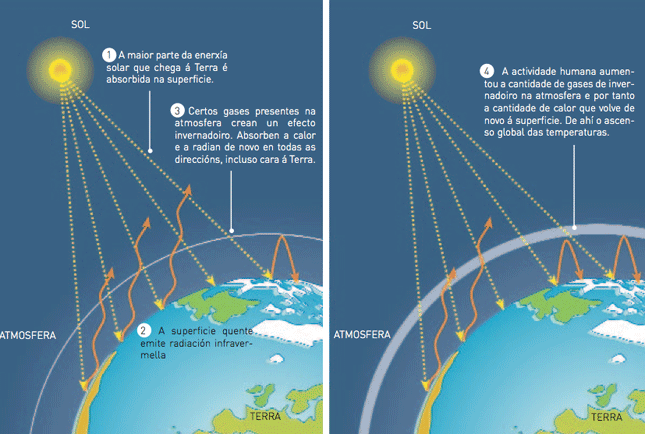
The greenhouse effect
That part of calorific energy retained by greenhouse gases is released again towards the Earth’s surface. This effect, known as greenhouse effect due to its similarity to what happens inside these agricultural facilities, warms the air surrounding the Earth. If there were not greenhouse gases, the planet would be about 30 degrees Celsius colder than it is at present.
In such conditions life would never be possible on Earth. This is what happens in Mars, where the temperature is -50 degrees Celsius. Nevertheless, these circumstances seem not to have been always the same since there seem to be morphological traces of riverside relief on it.
Relationship between the CO2 and the Earth’s temperature

After passing through all these atmospheric filters, about one-third of the solar radiation reaches the Earth. All the visible radiation that strikes the black soil is absorbed and the rest that strikes white objects is reflected (“rebounded off”). The radiation that strikes on objects of different colours is partly absorbed and partly reflected. This reflected illuminating radiation allows us to see the objects in the complementary colours of those absorbed.
Nevertheless, the part of illuminating energy absorbed is transformed into heat. This calorific energy is released into the atmosphere in the form of infrared radiation. Part of that heat released is trapped by the greenhouse gas layer.
As it can be inferred from above, the Earth is warmer due to the carbon dioxide contained -0.03 percent-, which acts as a calorific radiation filter, in a similar way to a glass wall of a greenhouse, since it is quite transparent for visible radiation but not so much for the infrared or calorific radiation.
Effects of the increasing CO2

If the content of carbon dioxide in the atmosphere was higher -0.06 percent-, the greenhouse effect would also be increased, warming the Earth several degrees, so that the polar caps could be gradually melting. The thicker the carbon dioxide layer is, the more similar to the glass wall of a greenhouse its behaviour is. If Arctic polar caps melted, the sea level would rise 72 metres.
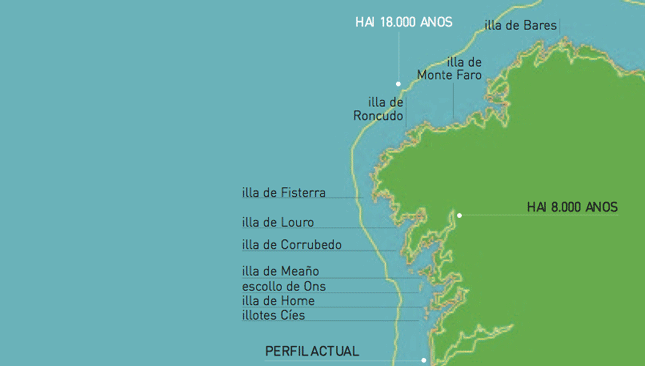
The opposite occurred as a consequence of the last ice age 18.000 years ago, when the sea level was 120 metres below the present level, so that the old coastline is covered by waters today. Eight thousand years ago there was a transgressive phase which raised the sea level by two meters above the actual coastline.
The role of CO2
Although the content of carbon dioxide (CO2) in the atmosphere represents 0.03 percent, nevertheless it is the main gas containing atmospheric carbon (C). Therefore, considering the atmospheric volume unit divided into one million parts, the CO2 would cover 358 parts –that is, the CO2 represents 358 parts per million-. The other two atmospheric gases containing carbon (C) are the methane (CH4) and carbon monoxide (CO), the first one standing for 0.1 parts/million and the second 1.6 parts/million.
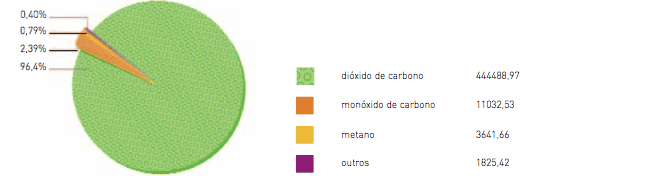
herefore, the carbon dioxide contains almost all the carbon in the atmosphere. But this gas is also found in the oceans and in the soil. There is a constant exchange of carbon between these three elements.
The carbon dioxide is one the chemical ways through which the carbon moves cyclically, so that the C released into the atmosphere from a source, returns again to that point, acting as a sink.
The main carbon reservoirs in the form of CO2 molecules that can be assimilated by living beings are found in the atmosphere and the hydrosphere.
The carbon cycle
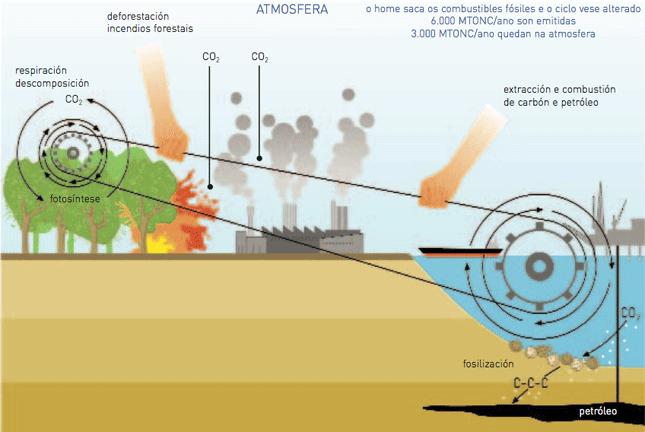
The carbon trapped from the atmosphere by living beings in the process of photosynthesis passes from some living beings to the other by means of the nutrition processes and it is released again into the atmosphere by means of respiration, bodies’ decomposition or burning, becoming a cycle. Some of these bodies does not decompose but become fossil inside the soil, getting rid of the carbon, since volcanoes, earthquakes, erosions and so on can release it again into the atmosphere in the form of CO2.
Then it can be said that the Earth’s carbon is on a constant move (carbon cycle). At the same time, this cycle can be divided into two adjusted sub-cycles produced at a different pace. This can be explained by means of the standard cycle path of the bicycle’s chain, as a result of the combination of the movement of the chain wheel and the sprocket wheel.
The quickest cycle (sprocket wheel) is related to the living beings’ and their bodies’ photosynthesis, nutrition and decomposition. The slowest cycle is geochemical since it is responsible for regulating the exchange between the soil and the atmosphere and it is related to the formation of fossil fuels from organic matter fossilization (carbon, natural gas and oil) and its burning or release as a result of geological processes).
The quick cycle and the role of photosynthesis as a sink
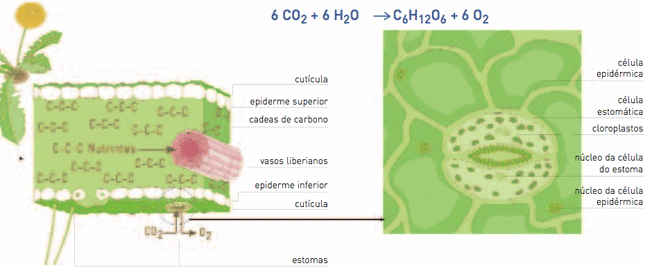
| Chloroplasts are inside the leaves. Photosynthesis is realized in them with the carbon dioxide coming from the air introduced through the stomata. This process takes place only if there is illuminating energy that is transformed into chemical energy stored in the linkages of G chains (nutrients) formed during the | process, using the C from the CO2. Oxygen going out into the atmosphere through the stomata is also released. Nutrients (C chains) dissolved into water become the juice that enters the Liberian vessels. |
Regarding the quick cycle, by means of photosynthesis biological processes introduce carbon dioxide from the atmosphere into organic compounds (carbon chains), which is stored in unions, the chemical energy resulting from the transformation of illuminating energy during the photosynthesis process. The gas goes into the plant through the leaves’ stomata (pores). The organic matter first produced is the carbon hydrates such as glucose, and from it the remaining substances are produced. The photosynthetic process can be summarized as follows:
Carbon dioxide occurs mainly in young forests. It is well-known that plants grow stronger in environments rich in carbon dioxide. As long as trees are growing, the relevance of respiration increases in order to maintain more biomass, keeping a balance between respiration and photosynthesis regarding carbon dioxide intake and release.
Forests in the quick cycle

In this sense, a forest ecosystem acts as a sink (a net atmospheric CO2 release), when there is an increasing sum of the total stocks retained by the same forest vegetation -trees and plants- in relation to the carbon dioxide released by that forest’s respiration and decomposition (the opposite to photosynthesis), as it occurs in young forests.
As far as the forest becomes mature these quantities of carbon dioxide source and sink become similar, reaching the balance. In this progress there can be the case of old forests where the CO2 release can overcome the CO2 sequestration.
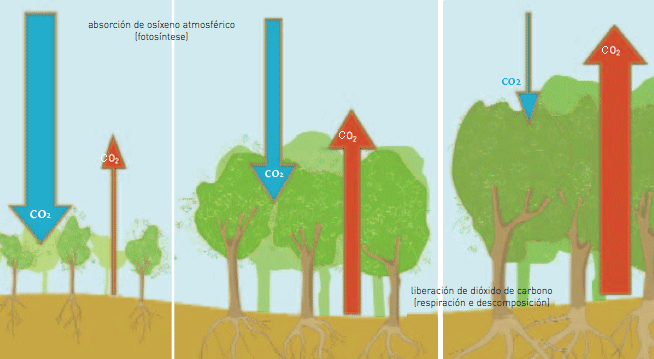
Considering that forests become older at the same time as the problems of deforestation and forest fires are increasing, the forests’ capacity to act as carbon dioxide sinks is limited, trapping no more that 20 percent of our emissions into the atmosphere.
Although the role of forests as CO2 sinks should not be dismissed, the key to achieve the removal of carbon dioxide surplus can be found in the sea, where the carbon dissolved is 50 times higher than that in the atmosphere. Therefore, the most important C sinks are found in the hydrosphere, mainly in the crustacean’s and molluscs carbonated shells as well as in the coralline formations.

| “The figure represents a forest in three moments of its evolution.
(a) On the left the forest is on a young stage. In this case it is acting as a carbon dioxide sink because it absorbs in photosynthesis more than it releases in respiration and decomposition. Therefore it is contributing to diminish the greenhouse effect. (b) In the middle it is on a mature stage. In this case the carbon dioxide intake and release are on a balance. Therefore, it is neither sink nor source and it does not affect the greenhouse effect. |
(c) On the right, there is an old forest where carbon dioxide emissions (source) overcome the absorptions (sink). Therefore it is contributing to the increase of the greenhouse effect. |
The slow cycle related to this issue
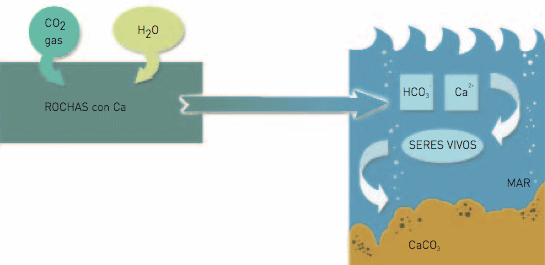
O dissolves rocks with calcium silicates from the continent (for example basalts), producing bicarbonates dissolved in water, deposited in the ocean. Here bicarbonates enter living being introducing the C from the atmosphere. Some of the living beings’ bodies are buried without being decomposed, becoming fossil as in the case of oil, or producing sedimentary rocks. These geological structures formed from living beings withdraw the C from the cycle.
Without the greenhouse gases’ layer, most of the Earth’s heat would go back to the Earth. This layer results from a natural balance achieved for a long time. With this layer the Earth has reached the necessary temperature for the development of life on it and thus the living beings managed to play a key role in keeping the balance to maintain this layer and the natural greenhouse effect necessary for life (carbon cycle).
Nevertheless, although these gases are good by themselves, too much carbon dioxide released by cars and power stations is causing a constant increase of temperature. This is due to the fact that these combustions derived from the human activity result from the extraction and burning of fossil fuels, accelerating artificially the cycle –artificial acceleration of the sprocket wheel-. Unbalances are taking place in the quick cycle (the chain wheel) due to human activities related to forest fires and deforestation.
This surplus of carbon dioxide released into the atmosphere by burning fossil fuels together with the effects of human activities on deforestation and forest fires mean that the carbon cycle is altered and that a surplus of CO2(g) is released into the atmosphere, increasing the thickness of the greenhouse gases layer.
While this layer is growing, it becomes similar to the glass since the greenhouse effect increases and this causes a constant rise of temperature. Most of the hottest twenty years recorded occurred after 1980. One of the main challenges faced by scientists in the 21st century is to stop the Earth’s atmosphere from changing this state of greenhouse into a state of natural heating.